Medicine details
| Image |  |
| Name | Uromax-D |
| Dosage | Capsule |
| Generic Name | Dutasteride + Tamsulosin Hydrochloride |
| Classes |
5-Alpha-Reductase Inhibitor |
| Diseases |
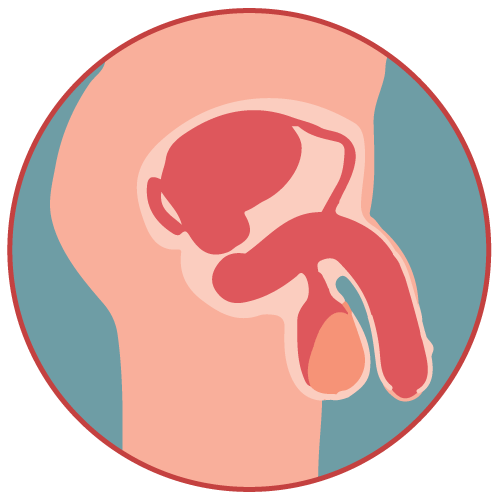
Enlarged Prostate Genito-Urinary Disease |
| Company | Unimed & Unihealth Manufacturers Ltd. |
Drug Package Details
| Strength | 500 mcg + 400 mcg |
| Storage Condition | |
| Origin Country | Bangladesh |
| Commercial Pack | 16 |
| Price per pack | ৳ 320.00 |
| Cost per pack | ৳ 281.60 |
| Package unit | 4 caps strip |
| Price per unit | ৳ 20.00 |
| Cost per unit | ৳ 17.60 |
| Discount | 0 |
| Coupon | |
| Remarks |
Dutasteride + Tamsulosin Hydrochloride
Dutasteride + Tamsulosin Hydrochloride is a combination medication that belongs to the class of drugs known as 5-alpha-reductase inhibitors and alpha-1 adrenergic blockers. Dutasteride inhibits the enzyme 5-alpha-reductase, which converts testosterone into dihydrotestosterone (DHT). By reducing DHT levels, dutasteride helps shrink the prostate gland. Tamsulosin is an alpha-1 adrenergic blocker that relaxes the muscles in the prostate and bladder neck, improving urine flow and relieving symptoms of benign prostatic hyperplasia (BPH).
- Dutasteride + Tamsulosin Hydrochloride is indicated for the treatment of symptomatic benign prostatic hyperplasia (BPH) in adult males.
- It is used to improve urinary symptoms associated with BPH, such as weak urine flow, incomplete bladder emptying, frequent urination, and urgency.
- Take one capsule daily approximately 30 minutes after the same meal each day.
- Swallow capsule whole.
- Each capsule contains 0.5 mg dutasteride and 0.4 mg tamsulosin hydrochloride.
The most common adverse reactions, reported in ≥1% of patients, treated with co-administered dutasteride and tamsulosin are-
- Ejaculation disorders (including decreased semen volume or absence of ejaculation).
- Impotence (erectile dysfunction).
- Dizziness.
- Orthostatic hypotension (low blood pressure upon standing).
- Headache.
- Nasal congestion.
- Rhinitis (inflammation of the nasal passages).
- Gastrointestinal disturbances (such as nausea, diarrhea, or constipation).
- Fatigue.
- Orthostatic hypotension: Dutasteride + Tamsulosin Hydrochloride may cause a sudden drop in blood pressure upon standing, resulting in dizziness or fainting. Caution should be exercised, especially when starting the medication or increasing the dose.
- Prostate cancer: Dutasteride + Tamsulosin Hydrochloride is not approved for the prevention of prostate cancer. It may reduce the detection of prostate cancer and may interfere with the interpretation of prostate-specific antigen (PSA) test results.
- Hepatic impairment: Use with caution in patients with liver dysfunction, as both dutasteride and tamsulosin are primarily metabolized in the liver.
- Ophthalmic complications during cataract surgery: Patients undergoing cataract surgery should inform their ophthalmologist about the use of Dutasteride + Tamsulosin Hydrochloride, as it may increase the risk of complications during surgery.
- Pediatric use: The safety and efficacy of this medication have not been established in pediatric patients.
Contraindication
Contraindicated in patients with previously demonstrated, clinically significant hypersensitivity (e.g., serious skin reactions, angioedema) to dutasteride, tamsulosin, other 5α-reductase inhibitors, or any component of the formulation.
None known.
Dutasteride + Tamsulosin is contraindicated in-
- Pregnancy and women of childbearing potential
- Pediatric patients



 Bangla
Bangla English
English