Medicine details
| Image |  |
| Name | Certican 0.50 |
| Dosage | Tablet |
| Generic Name | Everolimus |
| Classes |
Anticancer/Antineoplastic Agent Immunotherapeutic Agent Immunosuppressive Agent |
| Diseases |
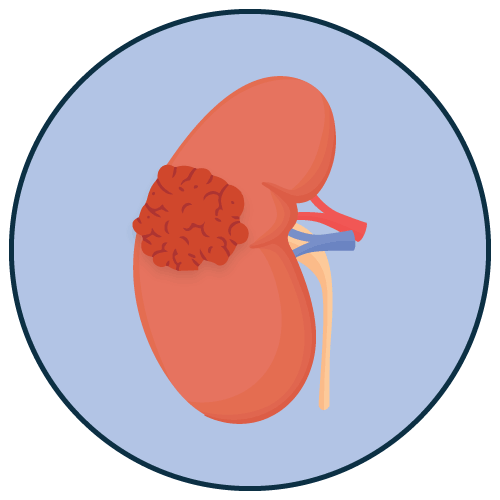
Cancer Kidney Cancer Tumor |
| Company | Novartis (Bangladesh) Ltd. |
Drug Package Details
| Strength | 0.50 mg |
| Storage Condition | |
| Origin Country | Bangladesh |
| Commercial Pack | 60 |
| Price per pack | ৳ 15,000.00 |
| Cost per pack | ৳ 13,200.00 |
| Package unit | 10 tabs strip |
| Price per unit | ৳ 250.00 |
| Cost per unit | ৳ 220.00 |
| Discount | 0 |
| Coupon | |
| Remarks |
Everolimus
Everolimus belongs to a class of drugs known as kinase inhibitors. Everolimus treats cancer by inhibiting cancer cell reproduction and reducing blood supply to cancer cells. Everolimus prevents transplant rejection by suppressing immune system activity.
Everolimus is indicated for the following conditions-
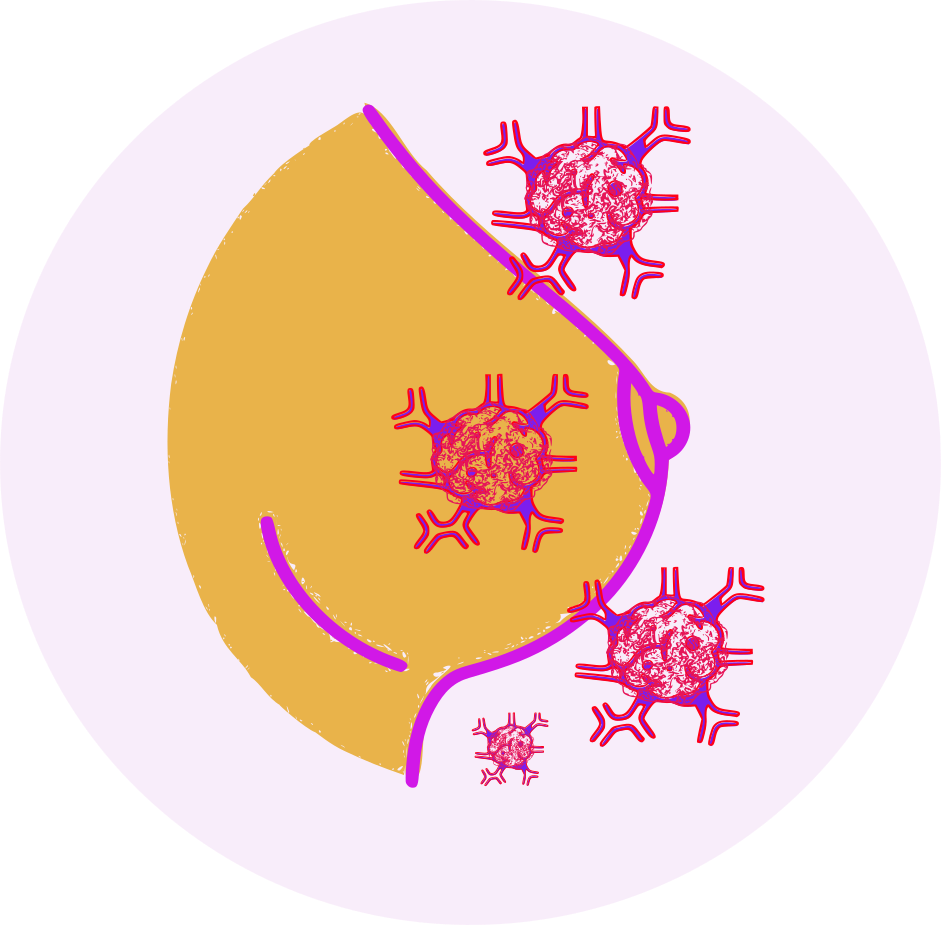
- Breast cancer
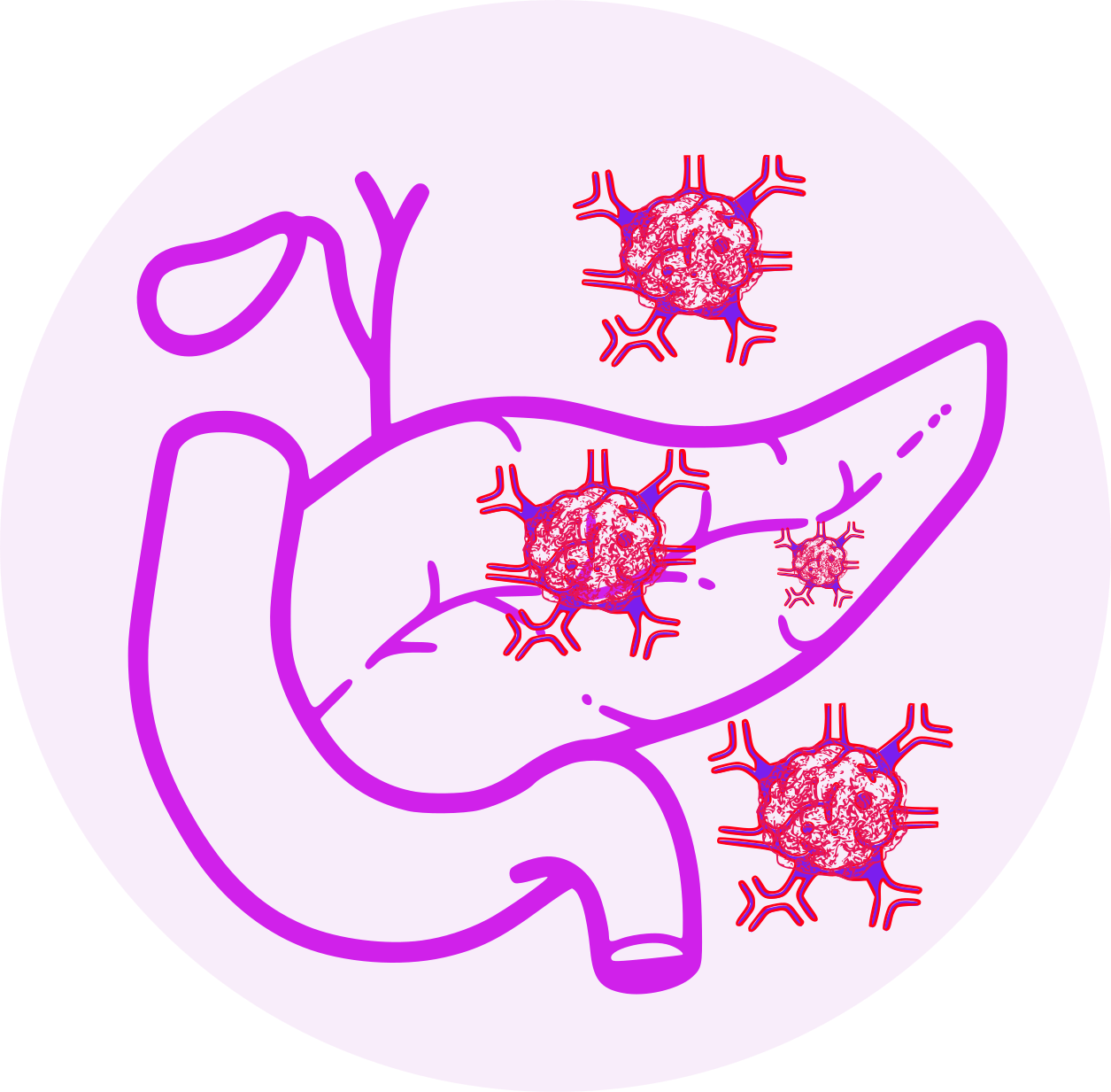
- Pancreatic tumors
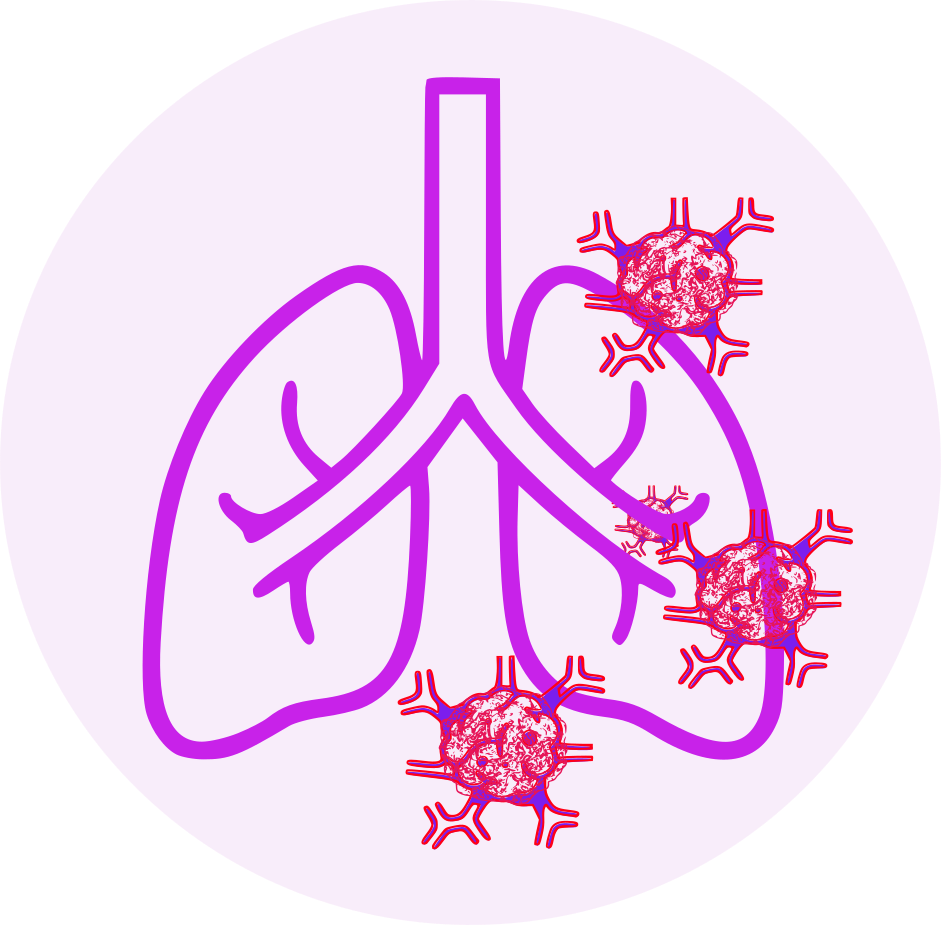
- lung cancer
- Renal cell carcinoma
- Everolimus Tablets are available in three dose regimens: 2.5 mg, 5 mg, and 10 mg.
- Everolimus 10 mg once daily is the recommended dose. Treatment should be continued for as long as there is clinical benefit or until unacceptable toxicity occurs.
- If a dose is missed, the patient should not take another dose, but should instead take the next prescribed dose as usual.
The following side effects have been commonly reported with the use of Everolimus-
- Infections
- Anemia
- Pancytopenia
- Anorexia
- Hyperglycemia
- Insomnia
- Eye edema
- Hemorrhage
- Shortness of breath
- Stomatitis
- Hepatic dysfunction
- Asthenia
- Rapamycin derivatives, including everolimus, can cause non-infectious pneumonitis. Non-infectious pneumonitis (including interstitial lung disease) has been reported frequently in Everolimus Tablets patients. Some cases were severe, and fatalities occurred on rare occasions. Non-infectious pneumonitis should be considered in patients who have non-specific respiratory signs and symptoms such as hypoxia, pleural effusion, cough, or dyspnea and have had infectious, neoplastic, and other non-medicinal causes ruled out by appropriate investigations.
- Patients who develop radiological changes suggestive of non-infectious pneumonitis but have few or no symptoms may continue to receive Everolimus Tablets without dose adjustments. Corticosteroids may be used until clinical symptoms resolve if they are moderate (Grade 2) or severe (Grade 3).
- Everolimus has immunosuppressive properties and may predispose patients to bacterial, fungal, viral or protozoan infections, including infections with opportunistic pathogens.
- Patients taking concomitant ACE inhibitor (e.g. ramipril) therapy may be at increased risk for angioedema (e.g. swelling of the airways or tongue, with or without respiratory impairment).
- Stomatitis, which includes mouth ulcers and oral mucositis, is the most commonly reported adverse reaction in patients receiving everolimus. Stomatitis is most common during the first 8 weeks of treatment. A single-arm study in postmenopausal breast cancer patients treated with everolimus plus exemestane found that using an alcohol-free corticosteroid oral solution as a mouthwash during the first 8 weeks of treatment may reduce the incidence and severity of stomatitis.
- Cases of renal failure (including acute renal failure), some fatal, have been reported in patients receiving everolimus.
Contraindication
Hypersensitivity to the active substance, to other rapamycin derivatives such as- temsirolimus, ridaforolimus or to any of the excipients.
None known.
None known.



 Bangla
Bangla English
English