Medicine details
| Image |  |
| Name | Eloxatin |
| Dosage | Injection |
| Generic Name | Oxaliplatin |
| Classes |
Anticancer/Antineoplastic Agent Alkylating Agent |
| Diseases |
Cancer |
| Company | Sanofi Aventis (BD) Ltd. |
Drug Package Details
| Strength | 50 mg |
| Storage Condition | |
| Origin Country | Bangladesh |
| Commercial Pack | 1 |
| Price per pack | ৳ 6,226.13 |
| Cost per pack | ৳ 5,478.99 |
| Package unit | 50 mg vial |
| Price per unit | ৳ 6,226.13 |
| Cost per unit | ৳ 5,478.99 |
| Discount | 0 |
| Coupon | |
| Remarks |
Oxaliplatin
Oxaliplatin is an anti neoplastic drug. The binding of Oxaliplatin to genomic DNA in the cell nucleus to create interstrand and intrastrand cross-links is the primary mechanism of the cytotoxic activity. This causes disruptions in the regular transcription and/or DNA replication processes, which sets off cytotoxic reactions that result in cell death.
Oxaliplatin is a platinum-based drug used in combination with infusional fluorouracil and leucovorin, which is indicated for:
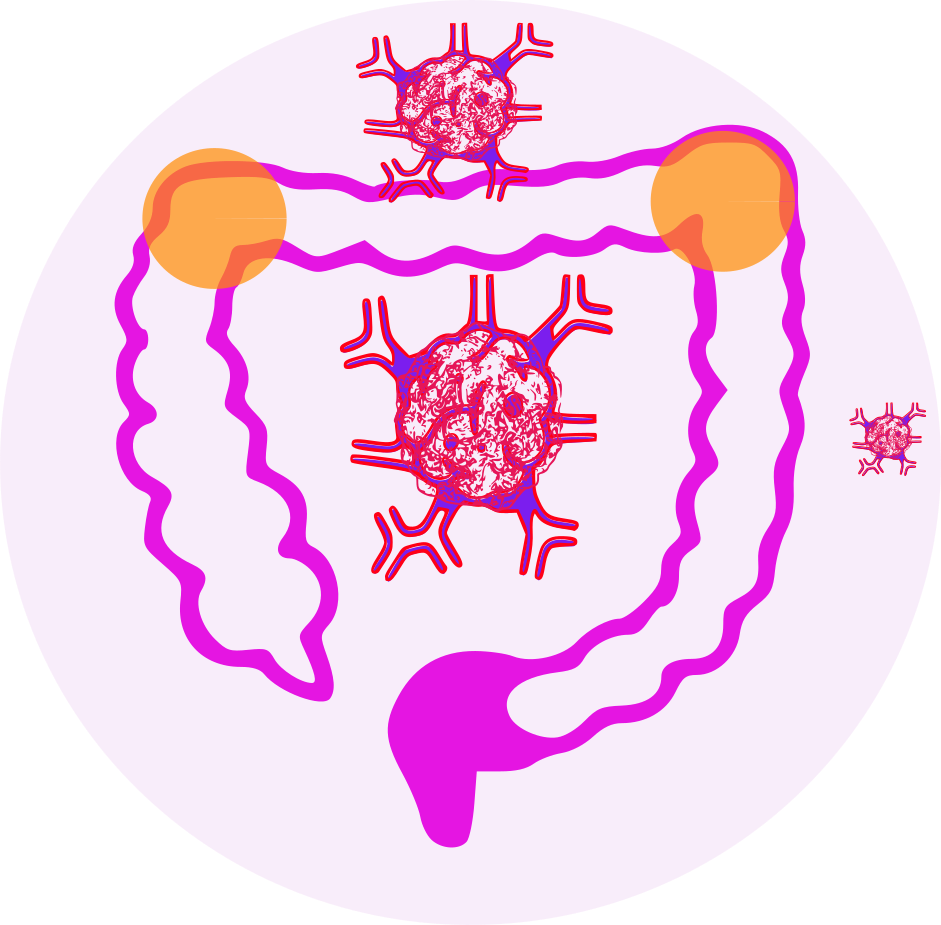
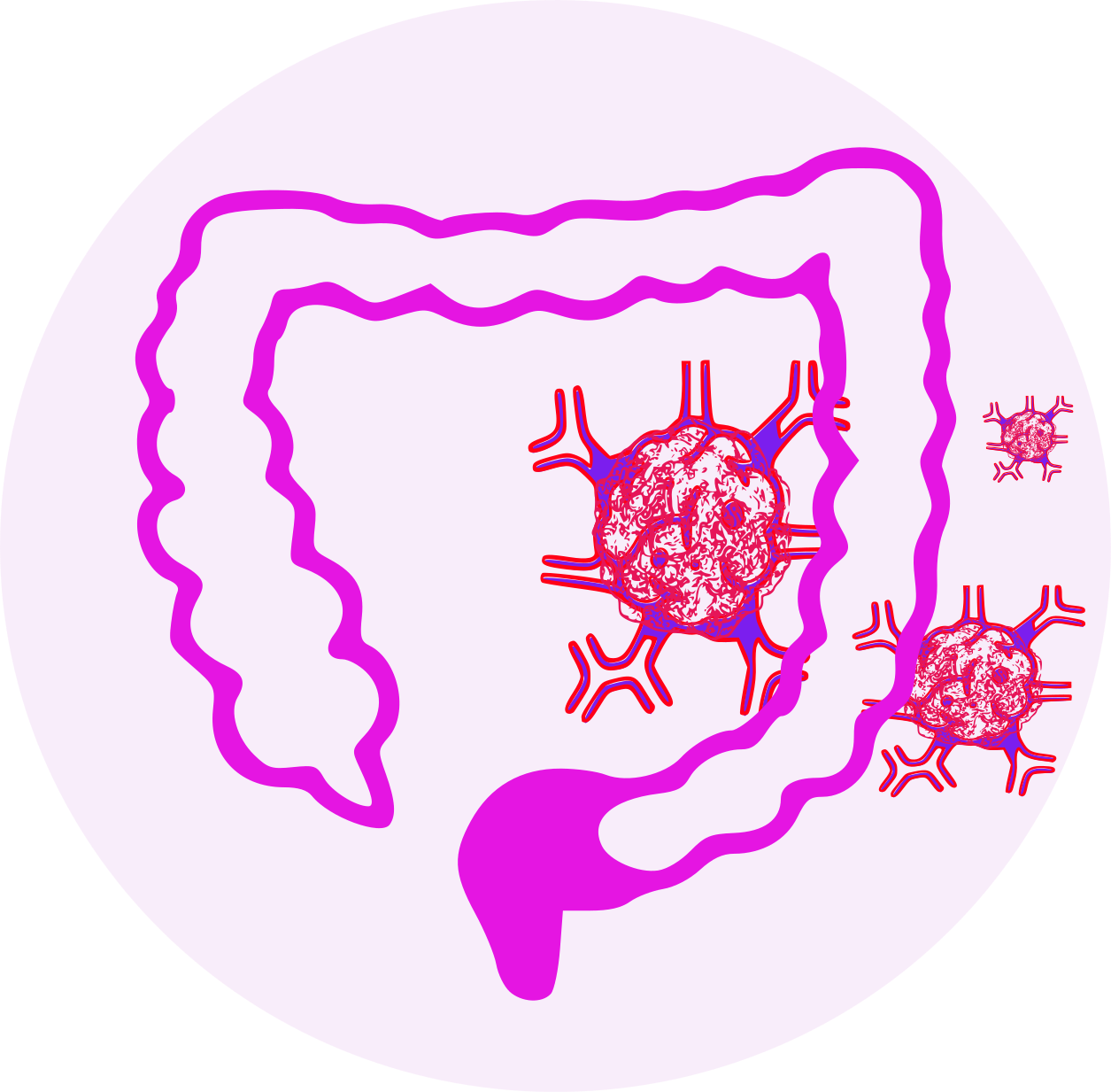
- adjuvant treatment of stage III colon cancer in patients who have undergone complete resection of the primary tumor.
- treatment of advanced colorectal cancer
- Administer Oxaliplatin 85 mg/m2 as an intravenous infusion over 120 minutes concurrently with leucovorin over 120 minutes in separate bags, followed by fluorouracil on Day 1 of each 14-day cycle. Administer fluorouracil and leucovorin on Day 2 as recommended.
- Adjuvant Treatment: Continue treatment for up to 12 cycles or unacceptable toxicity.
- Advanced Colorectal Cancer: Continue treatment until disease progression or unacceptable toxicity.
Most common adverse reactions were-
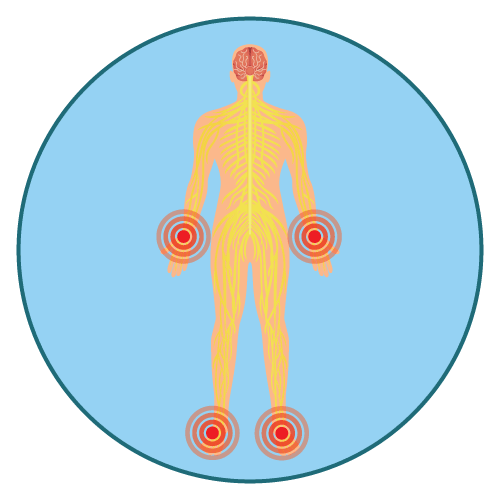
- peripheral sensory neuropathy
- neutropenia
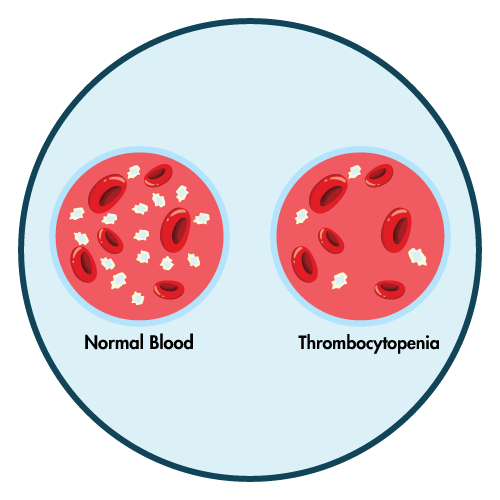
- thrombocytopenia
- anemia
- nausea
- increase in transaminases and alkaline phosphatase
- diarrhea
- emesis
- fatigue
- stomatitis
- Hypersensitive reactions: Serious and fatal hypersensitivity adverse reactions, including anaphylaxis, can occur with Oxaliplatin within minutes of administration and during any cycle. Oxaliplatin is contraindicated in patients with hypersensitivity reactions to oxaliplatin and other platinum-based drugs. Immediately and permanently discontinue Oxaliplatin for hypersensitivity reactions and administer appropriate treatment.
- Peripheral Sensory Neuropathy: Acute and delayed neuropathy are both possible. Ice should not be applied topically. Reduce the dose or discontinue Oxaliplatin completely as directed.
- Severe Myelosuppression: Delay Oxaliplatin until neutrophils and platelets are greater than or equal to 1.5 109/L and 75 109/L, respectively. If you have sepsis or septic shock, you should not take Oxaliplatin. Reduce the dose as recommended after recovering from grade 4 neutropenia, febrile neutropenia, or grade 3-4 thrombocytopenia.
- Posterior Reversible Encephalopathy Syndrome (PRES): In patients who develop PRES, discontinue Oxaliplatin permanently.
- Pulmonary Toxicity: Withhold Oxaliplatin until interstitial lung disease or pulmonary fibrosis has been ruled out.
- QT Interval Prolongation: In patients with congenital long QT syndrome, avoid. Electrocardiograms should be monitored in patients with congestive heart failure, bradyarrhythmias, and electrolyte abnormalities, as well as in patients taking drugs that are known to prolong the QT interval. Correct electrolyte imbalances before starting Oxaliplatin and on a regular basis during treatment.
- Hepatotoxicity: Monitor liver function tests at baseline, before each subsequent cycle, and as clinically indicated.
- Rhabdomyolysis: Permanently discontinue Oxaliplatin if rhabdomyolysis occurs.
- Hemorrhage: Increase frequency of monitoring in patients who are receiving Oxaliplatin with fluorouracil/leucovorin and oral anticoagulants.
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise pregnant women of the potential risk to a fetus. Advise males and females of reproductive potential to use an effective method of contraception.
Contraindication
Patients with hypersensitivity reaction to oxaliplatin or other platinum-based drugs such as-
None known.
Cisplatin is contraindicated in pregnancy.




 Bangla
Bangla English
English