| Name | Rivastigmine |
| Classes |
Central Nervous System Agent AntiAlzheimer Agent Cholinesterase Inhibitor |
| Diseases |
Alzheimer's Disease CNS Disorder Dementia Parkinson's Disease |
Rivastigmine
Rivastigmine is an acetylcholinesterase inhibitor. Acetylcholinesterase is the enzyme responsible for breaking down acetylcholine. Therefore, by blocking the enzyme, Rivastigmine increases the acetylcholine concentration in the synaptic cleft or neuro-muscular junction.
Rivastigmine is indicated for treatment of-
- Mild-to-moderate dementia of the Alzheimer’s type
- Mild-to-moderate dementia associated with Parkinson’s disease
Alzheimer's Disease:
- In Alzheimer's disease (AD), the recommended dose of Rivastigmine Oral Solution and Rivastigmine Capsules is 6 mg to 12 mg twice daily (daily doses of 3 mg to 6 mg twice a day). Clinical trials have shown that doses at the higher end of this range may be more beneficial.
- Initial Dose: Begin treatment with 1.5 mg of Rivastigmine twice a day.
- Dose Adjustment: Increase the dose to 3 mg twice a day after a minimum of two weeks and if well tolerated. Following a minimum of 2 weeks at the previous dose and if well tolerated, subsequent increases to 4.5 mg twice daily and 6 mg twice daily should be attempted. The maximum dose is 6 mg twice a day (12 mg per day).
Parkinson’s Disease Dementia:
- Rivastigmine should be taken with meals in divided doses in the morning and evening.
- Rivastigmine was shown to be effective in a single controlled clinical trial in dementia associated with Parkinson's disease at doses ranging from 3 mg to 12 mg twice daily (daily doses of 1.5 mg to 6 mg twice a day).
- Initial Dose: Initiate treatment with the 1.5 mg twice a day with Rivastigmine.
- Dose Titration: Increase the dose to 3 mg twice a day after a minimum of 4 weeks and if well tolerated. Following a minimum of 4 weeks at the previous dose and if well tolerated, subsequent increases to 4.5 mg twice daily and 6 mg twice daily should be attempted. The maximum daily dose is 6 mg taken twice a day (12 mg per day).
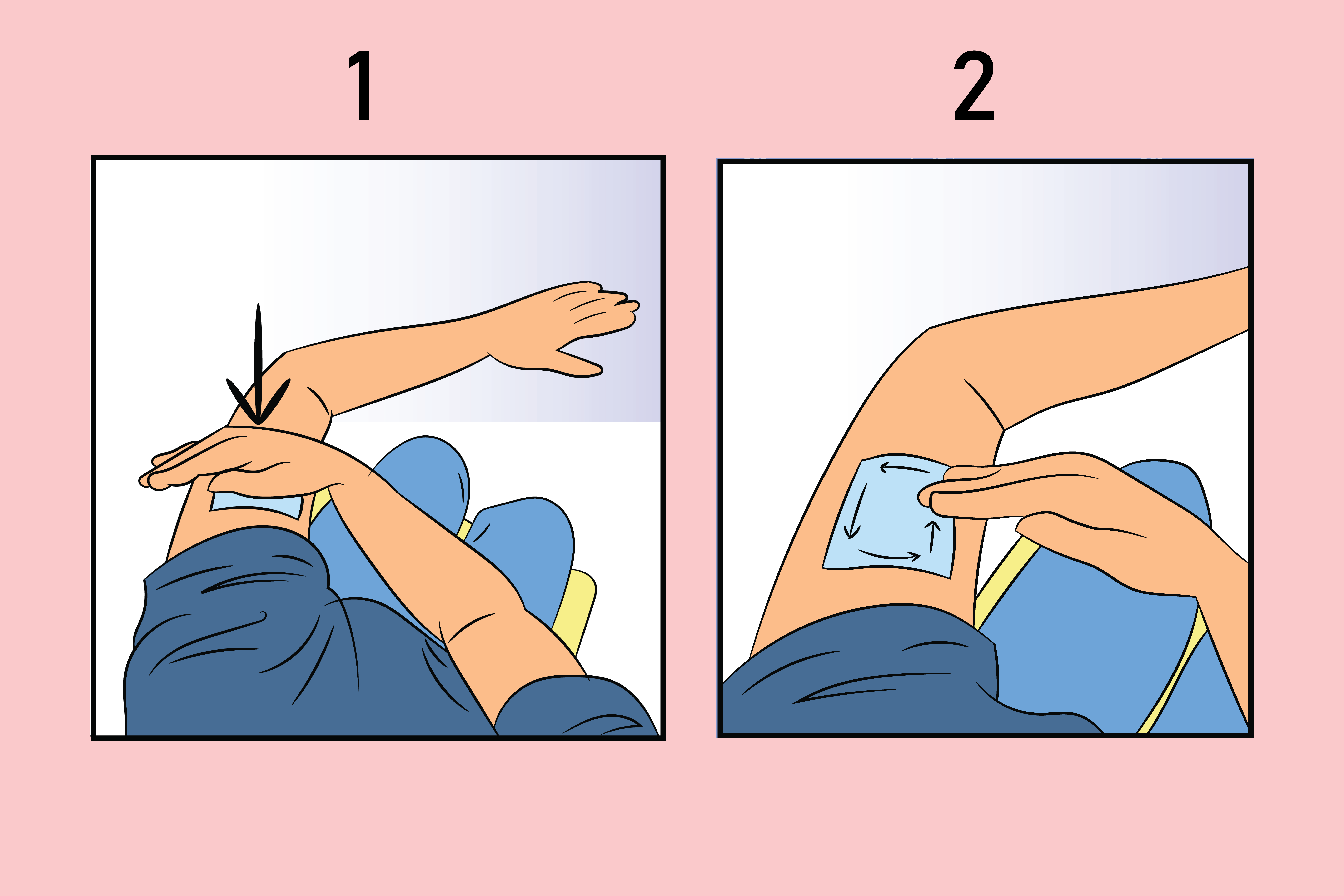
Rivastigmine is also available as transdermal patch.
- Initial Dose: one Exelon Patch 4.6 mg/24 hours once daily
- Maintenance Dose: one Exelon Patch 9.5 mg/24 hours once daily
A minimum of 4 weeks of treatment and good tolerability with the previous dose should be observed before increasing the dose.
| Administering Transdermal Patch |
- Significant nausea, vomiting, diarrhea, anorexia/low appetite, and weight loss are examples of gastrointestinal adverse reactions that may necessitate treatment discontinuation. Dehydration can occur as a result of prolonged vomiting or diarrhea and can have serious consequences.
- In the event of disseminated allergic dermatitis following oral or transdermal administration, discontinue rivastigmine. Switch to oral rivastigmine only after negative allergy testing in patients with suspected allergic contact dermatitis after transdermal rivastigmine use.
Contraindication
Known hypersensitivity to rivastigmine, other carbamate derivatives or other components of the formulation.
None known.
History of application site reaction with rivastigmine transdermal patch suggestive of allergic contact dermatitis, in the absence of negative allergy testing.
 Bangla
Bangla English
English