| Name | Testosterone Undecanoate |
| Classes |
Hormonal Agent Sex Hormone Steroid |
| Diseases |
Hormonal Disorder Hypogonadism Testosterone Replacement Therapy |
Testosterone Undecanoate
Testosterone is a male sex hormone produced in the testicles. Testosterone is responsible for normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include growth and maturation of the prostate, seminal vesicles, penis, and scrotum; development of male hair distribution, such as beard, pubic, chest, and axillary hair; laryngeal enlargement, vocal cord thickening, and alterations in body musculature and fat distribution.
The use of testosterone injection as a replacement therapy in males is advised in cases where there are signs of endogenous testosterone insufficiency or absence.
- Primary hypogonadism (congenital or acquired)-testicular failure due to cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome; or orchidectomy.
- Hypogonadotropic hypogonadism (congenital or acquired)- gonadotropin or LHRH deficiency, or pituitary-hypothalamic injury from tumors, trauma, or radiation.
- Prior to initiating Testosterone Undecanoate, confirm the diagnosis of hypogonadism by ensuring that serum testosterone concentrations have been measured in the morning on at least two separate days and that these concentrations are below the normal range.
- Take Testosterone Undecanoate with food.
- Starting dose: 237 mg orally once in the morning and once in the evening.
- Adjust the dose to a minimum of 158 mg twice daily and a maximum of 396 mg twice daily based on serum testosterone drawn 6 hours after the morning dose at least 7 days after starting treatment or following dose adjustment and periodically thereafter.
Most common adverse reactions associated with testosterone therapy include-
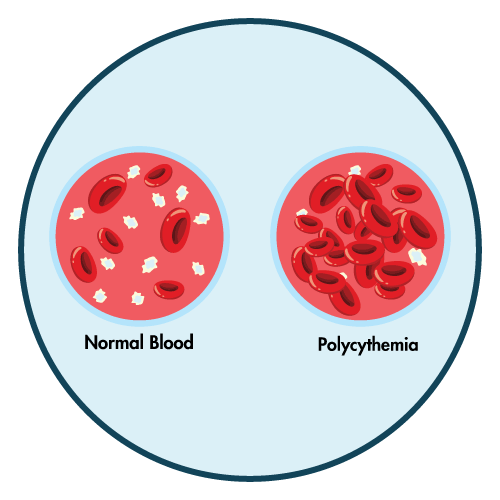
- polycythemia
- diarrhea
- dyspepsia
- eructation
- peripheral edema
- nausea
- increased hematocrit
- headache
- prostatomegaly
- hypertension
- To identify polycythemia and increasing red blood cell mass, check the hematocrit every three months.
- Patients with benign prostatic hyperplasia (BPH) should be monitored for worsening BPH symptoms and signs.
- Patients using testosterone have been known to experience venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE). Patients who exhibit symptoms or signs of PE or DVT should be evaluated.
- The most common ways that testosterone has been abused are in combination with other anabolic androgenic drugs and at levels that are greater than those permitted for usage.
- Aazoospermia may result after the administration of androgens exogenously.
- Patients with underlying cardiac, renal, or hepatic illness may experience edema, either with or without congestive heart failure.
- Sleep apnea may occur in those with risk factors.
- Monitor prostate specific antigen (PSA) and lipid concentrations periodically.
- Depression and suicidal ideation have occurred during clinical trials in patients treated with Testosterone.
Contraindication
Contraindicated in patients hypersensitive to testosterone or any of it's ingreients.
None known.
Contraindicated in-
- Men with breast cancer or known or suspected prostate cancer
- Men with hypogonadal conditions not associated with structural or genetic etiologies
- Pregnancy
 Bangla
Bangla English
English