| Name | Danazol |
| Classes |
Hormonal Agent Antigonadotropic Agent |
| Diseases |
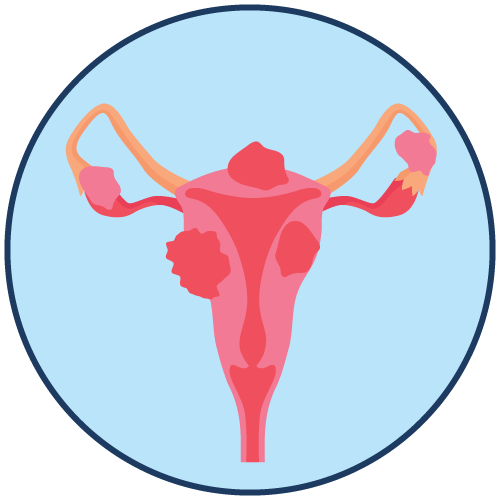
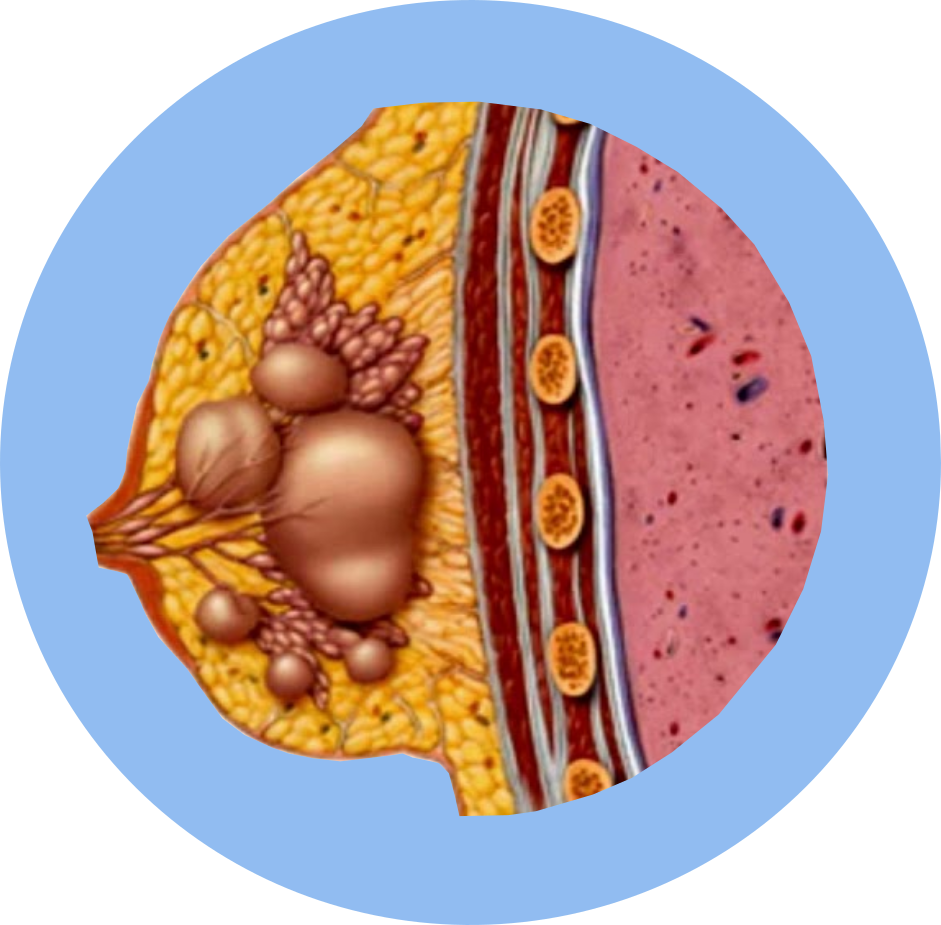
Breast Fibrocystic Inflammatory Disease Swelling Uterus Pain |
Danazol
Danazol is classified as a synthetic androgen. Its mechanism of action involves suppression of gonadotropin secretion, leading to inhibition of ovarian and testicular steroidogenesis. Additionally, Danazol exerts anti-estrogenic effects by inhibiting the action of estrogen on target tissues. This combination of actions results in a decrease in the size and function of endometrial tissue and a reduction in the symptoms associated with certain hormonal conditions.
Danazol is indicated for the treatment of:
- Endometriosis: Relief of symptoms and reduction of endometrial lesions.
- Fibrocystic Breast Disease: Management of severe cases.
- Hereditary Angioedema: Prevention of attacks in both males and females.
Dosage recommendations may vary based on the specific indication and individual patient response.
-
Endometriosis:
- Initial dose: 800 mg to 1200 mg daily in two divided doses.
- Maintenance dose: Adjusted based on response, typically 200 mg to 800 mg daily.
-
Fibrocystic Breast Disease:
- Initial dose: 200 mg to 400 mg twice daily.
- Maintenance dose: Adjusted based on response.
-
Hereditary Angioedema:
- Initial dose: 200 mg to 600 mg daily in two divided doses.
- Maintenance dose: Adjusted based on response.
Adverse reactions associated with Danazol are listed from most common to least common:
- Weight gain
- Edema
- Acne
- Oily skin
- Irregular menses
- Hot flashes
- Sweating
- Voice changes
- Decreased breast size
- Headache
- Liver Function: Monitor liver function regularly, as Danazol may cause hepatotoxicity. Discontinue if significant liver enzyme elevations occur.
- Androgenic Effects: Danazol has androgenic properties. Assess androgenic effects, and adjust dosage if virilization occurs.
- Pregnancy Category X: Danazol is contraindicated in pregnancy due to potential fetal harm. Effective contraception is essential during and for at least 12 weeks after Danazol therapy.
- Lipid Changes: Monitor lipid profiles regularly, as Danazol may cause changes in serum lipids.
- Benign Intracranial Hypertension: Monitor for signs of benign intracranial hypertension, especially in patients with a history of pseudotumor cerebri.
Contraindication
Contraindicated in patients with hypersensitivity to danazol.
None known.
Danazol is contraindicated in patients with-
- Undiagnosed abnormal genital bleeding.
- Markedly impaired hepatic, renal, or cardiac function.
- Pregnancy.
- Breast feeding.
- Porphyria
- Androgen-dependent tumor.
- Active thrombosis or thromboembolic disease and history of such events.
 Bangla
Bangla English
English