| Name | Nelfinavir |
| Classes |
Antiinfective Agent Antiviral Agent Protease Inhibitor |
| Diseases |
HIV Infectious Disease |
Nelfinavir
Nelfinavir is an antiviral drug from the class protease inhibitor. The HIV-1 protease is inhibited by the peptidomimetic drug Nelfinavir. HIV protease inhibition prevents the enzyme from processing the Gag-Pol polyprotein precursor, which results in the generation of immature, non-infectious HIV particles.
Nelfinavir is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection.
- Adults: 1250 mg (five 250 mg tablets or two 625 mg tablets) twice daily or 750 mg (three 250 mg tablets) three times daily is the recommended dose. VIRACEPT must be taken with food. Patients who are unable to swallow the 250 or 625 mg tablets should dissolve them in a small amount of water. Patients should thoroughly mix the cloudy liquid after it has been dissolved and consume it right away. To ensure that the entire dose is consumed, the glass should be rinsed with water and the rinse swallowed.
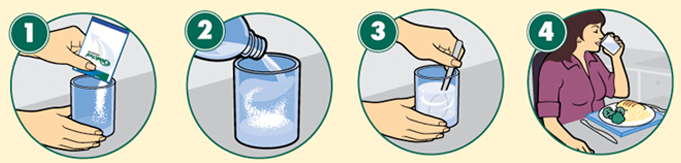
- Pediatric Patients (2-13 years): The recommended oral dose of VIRACEPT Oral Powder or 250 mg tablets in children 2 years of age and older is 45 to 55 mg/kg twice daily or 25 to 35 mg/kg three times daily. All doses must be taken with food. VIRACEPT Oral Powder can be given to children who are unable to take tablets. To obtain the full dose, the oral powder should be mixed with a small amount of water, milk, formula, soy formula, soy milk, or dietary supplements; once mixed, the entire contents must be consumed. If the mixture is not consumed immediately, it must be refrigerated and stored for no more than 6 hours. Acidic food or juice (e.g., orange juice, apple juice, or apple sauce) are not recommended to be used in combination with VIRACEPT, because the combination may result in a bitter taste. VIRACEPT Oral Powder should not be reconstituted with water in its original container.
| How to administer oral powder: |
The most frequently reported adverse drug reactions were-
- Diarrhea
- Nausea
- Flatulence
- Rash
- leukopenia
- myalgia
- Hemophilia: There have been reports of increased bleeding, including spontaneous skin hematomas and hemarthrosis, in patients with hemophilia type A and B treated with protease inhibitors. In some patients, additional factor VIII was given. In more than half of the reported cases, treatment with protease inhibitors was continued or reintroduced. A causal relationship has not been established.
- Fat Redistribution: Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (“buffalo hump”), peripheral wasting, facial wasting, breast enlargement, and “cushingoid appearance” have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
- Immune Reconstitution Syndrome: Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including Nelfinavir. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia (PCP), or tuberculosis), which may necessitate further evaluation and treatment.
- Resistance/Cross Resistance: HIV cross-resistance between protease inhibitors has been observed.
Contraindication
- Contraindicated in patients with clinically significant hypersensitivity to any of its components.
- Co-administration with the following drugs is contraindicated-
-
None known.
None known.
 Bangla
Bangla English
English