| Name | Gemcitabine |
| Classes |
Anticancer/Antineoplastic Agent Antimetabolites |
| Diseases |
Breast Cancer Cancer Lung Cancer Ovarian Cancer Pancreatic Cancer |
Gemcitabine
Gemcitabine is an antineoplastic drug from the class of antimetabolites. Action Mechanism Gemcitabine kills cells that are undergoing DNA synthesis and prevents cells from progressing through the G1/S phase boundary. Nucleoside kinases convert gemcitabine to diphosphate (dFdCDP) and triphosphate (dFdCTP) nucleosides. Gemcitabine diphosphate inhibits ribonucleotide reductase, an enzyme that catalyzes the reactions that generate deoxynucleoside triphosphates for DNA synthesis, causing deoxynucleotide concentrations to fall.
Gemcitabine is a nucleoside metabolic inhibitor indicated:
- in combination with carboplatin, for the treatment of advanced ovarian cancer that has relapsed at least 6 months after completion of platinum based therapy
- in combination with paclitaxel, for first-line treatment of metastatic breast cancer after failure of prior anthracycline-containing adjuvant chemotherapy, unless anthracyclines were clinically contraindicated
- in combination with cisplatin for the treatment of non-small cell lung cancer
- as a single agent for the treatment of pancreatic cancer
- Ovarian Cancer: 1000 mg/m2 over 30 minutes on Days 1 and 8 of each 21-day cycle
- Breast Cancer: 1250 mg/m2 over 30 minutes on Days 1 and 8 of each 21 day cycle
- Non-Small Cell Lung Cancer: 1000 mg/m2 over 30 minutes on Days 1, 8, and 15 of each 28-day cycle or 1250 mg/m2 over 30 minutes on Days 1 and 8 of each 21-day cycle
- Pancreatic Cancer: 1000 mg/m2 over 30 minutes once weekly for the first 7 weeks, then one week rest, then once weekly for 3 weeks of each 28-day cycle.
The most common adverse reactions associated with gemcitabine are-
- nausea/vomiting
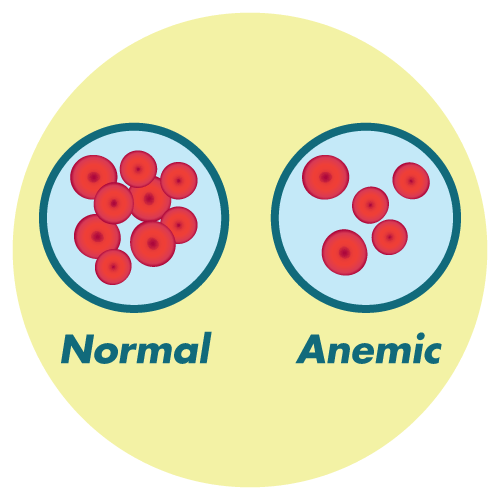
- anemia
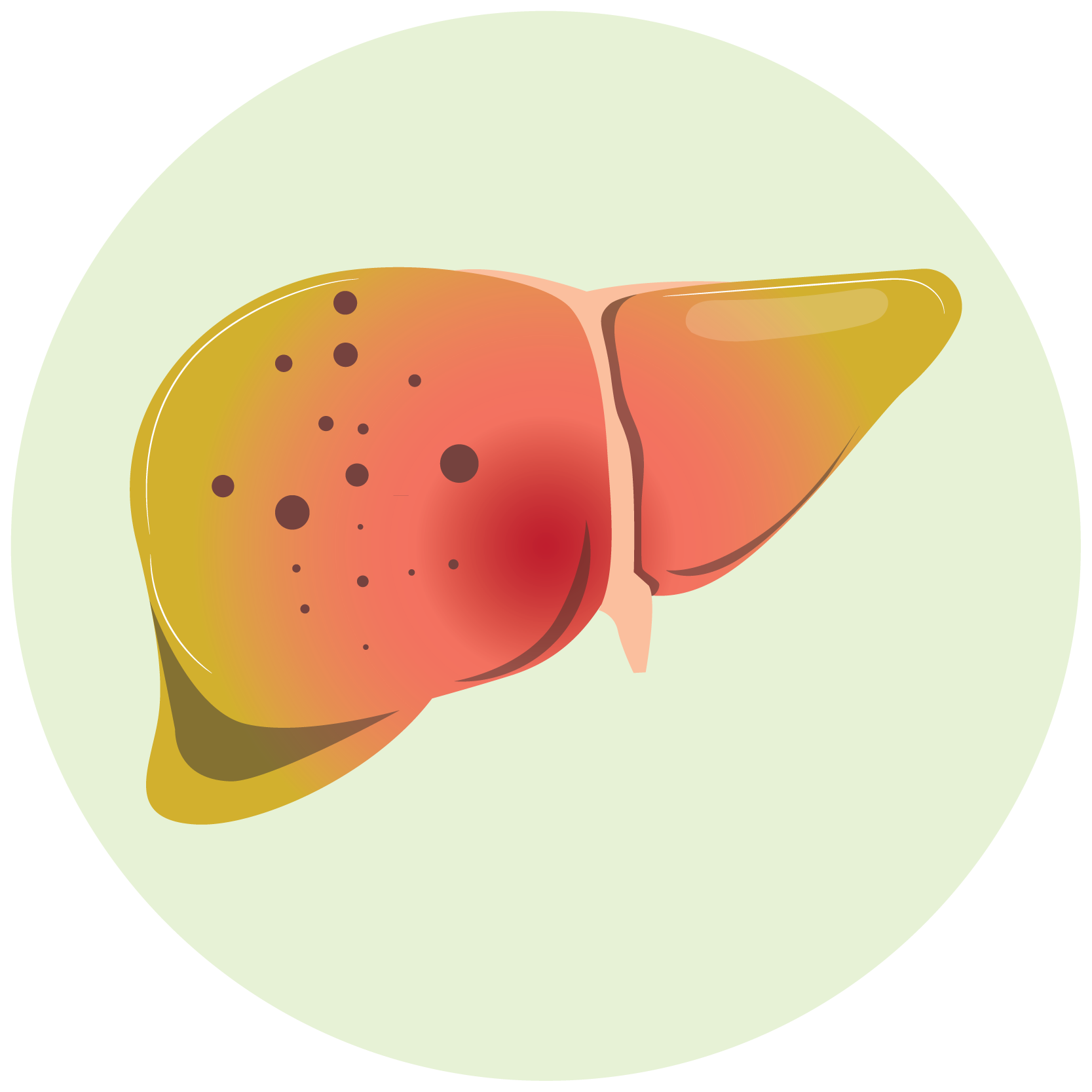
- hepatic transaminitis
- neutropenia
- increased alkaline phosphatase
- proteinuria
- fever
- hematuria
- rash
- thrombocytopenia
- dyspnea
- peripheral edema
- Schedule-dependent toxicity: Increased toxicity with infusion time greater than 60 minutes or dosing more frequently than once weekly.
- Myelosuppression: Monitor for myelosuppression prior to each cycle and reduce or withhold dose for severe myelosuppression.
- Pulmonary Toxicity and Respiratory Failure: Discontinue Gemcitabine immediately for unexplained new or worsening dyspnea or evidence of severe pulmonary toxicity.
- Hemolytic-Uremic Syndrome (HUS): Monitor renal function prior to initiation and during therapy. Discontinue Gemcitabine for HUS or severe renal impairment.
- Hepatic Toxicity: Monitor hepatic function prior to initiation and during therapy. Discontinue Gemcitabine for severe hepatic toxicity.
- Embryofetal Toxicity: Can cause fetal harm. Advise women of potential risk to the fetus.
- Exacerbation of Radiation Therapy Toxicity: May cause severe and life threatening toxicity when administered during or within 7 days of radiation therapy.
- Capillary Leak Syndrome: Discontinue Gemcitabine.
- Posterior reversible encephalopathy syndrome (PRES): Discontinue Gemcitabine.
Contraindication
Contraindicated in patients with a known hypersensitivity to gemcitabine.
None known.
None known.
 Bangla
Bangla English
English