| Name | Ritonavir |
| Classes |
Antiinfective Agent Antiviral Agent Protease Inhibitor |
| Diseases |
HIV Infectious Disease |
Ritonavir
Ritonavir is an antiviral drug from the class protease inhibitor. The HIV-1 protease is inhibited by the peptidomimetic drug ritonavir. HIV protease inhibition prevents the enzyme from processing the Gag-Pol polyprotein precursor, which results in the generation of immature, non-infectious HIV particles.
Ritonavir is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection.
- Recommended Adult Dosage: Ritonavir should be taken orally twice day with meals in doses of 600 mg each. Starting with no less than 300 mg twice daily, Ritonavir should be raised by 100 mg twice daily every two to three days. After the titration is over, the maximum dose of 600 mg twice a day should not be exceeded.
- Recommended Pediatric Dosage: Pediatric patients older than one month are advised to take Ritonavir at a dose of 350 to 400 mg per m2 taken orally twice daily with meals, with a maximum dose of 600 mg twice daily. Starting with 250 mg per m2 twice daily, Ritonavir should be raised by 50 mg per m2 twice daily every two to three days. The greatest tolerable dose may be used for maintenance therapy in conjunction with other antiretroviral drugs, however other therapies should be taken into consideration if patients cannot tolerate 400 mg per m2 twice daily due to side effects.
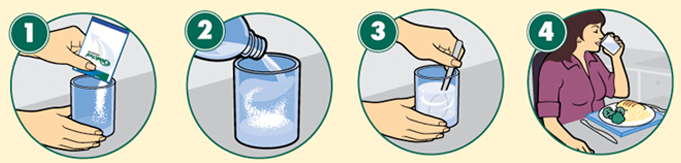
- Preparation of Ritonavir Oral Powder: Prepare the dose with the appropriate number of packets. Use one packet for 100 mg doses and two packets for 200 mg doses, for example. Pour the entire contents of each packet over soft food or liquid and mix thoroughly. All powder should be administered within 2 hours of being mixed with soft food or liquid. If the mixture is not administered within 2 hours of preparation, it should be discarded and a new dose prepared. After being mixed with water, the prescribed dose of Ritonavir oral powder can be administered via a feeding tube. To administer the medication, follow the feeding tube instructions.
| Preparation of oral powder |
The most frequently reported adverse drug reactions were-
- diarrhea
- nausea
- vomiting
- abdominal pain
- paresthesia and oral paresthesia
- rash
- fatigue/asthenia
- The use of Ritonavir in conjunction with certain other drugs may result in known or potentially significant drug interactions. For potential drug interactions, consult the full prescribing information before and during treatment.
- Because of the possibility of toxicity, Ritonavir oral solution should not be used in preterm neonates in the immediate postnatal period. In this patient population, a safe and effective dose of Ritonavir oral solution has not been established.
- There have been fatalities. Monitor liver function before and during treatment, especially in patients with underlying hepatic disease, such as hepatitis B or C, or significant transaminase elevations. There have been fatalities; discontinue therapy as clinically appropriate.
- Anaphylaxis, toxic epidermal necrolysis, Stevens-Johnson syndrome, bronchospasm, and angioedema have all been reported as allergic reactions. If severe reactions occur, discontinue treatment.
- Some patients may experience PR interval prolongation. There have been reports of second and third degree heart block. When used in patients with preexisting conduction system disease, ischemic heart disease, cardiomyopathy, underlying structural heart disease, or when combined with other drugs that may prolong the PR interval, use with caution.
- Total cholesterol and triglycerides elevations: Monitor prior to therapy and periodically thereafter.
- Patients may develop new onset or exacerbations of diabetes mellitus, hyperglycemia.
- Immune reconstitution syndrome may develop in patients.
- Patients may experience fat redistribution/accumulation.
- Spontaneous bleeding may occur, necessitating the use of additional factor VIII.
Contraindication
- Ritonavir is contraindicated in patients with known hypersensitivity to ritonavir or any of its ingredients.
- Co-administration with drugs highly dependent on CYP3A for clearance and for which elevated plasma concentrations may be associated with serious and/or life-threatening events. Examples of such drugs are-
None known.
None known.
 Bangla
Bangla English
English